Fox news reporter, Stacy Case, delivers an emotional testimony at the FDA's townhall in California, Septe ...
-
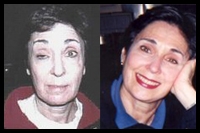
Fox news reporter Stacy Case presents a powerful testimonial of her mercury induced MS to the FDA
Fox news reporter Stacy Case presents a powerful testimonial of her mercury induced MS to the FDA
-
James Hardy DMD presents his testimony to the FDA’s CDRH Townhall in Orlando March 2011
James Hardy DMD presents his testimony to the FDA’s CDRH Townhall in Orlando March 2011
-
Injured Consumer Freya Koss Testifies at the FDA Townhall in Orlando
Injured Consumer Freya Koss Testifies at the FDA Townhall in Orlando
-
Floridians tell Dr. Shuren of the FDA’s CDRH – BAN MERCURY AMALGAM NOW!
Floridians tell Dr. Shuren of the FDA’s CDRH – BAN MERCURY AMALGAM NOW!
-
Testimony from DAMS President at FDA townhall in Orlando
Testimony from DAMS President at FDA townhall in Orlando
-
FDA gets an earful about dental amalgam at town hall meeting
FDA gets an earful about dental amalgam at town hall meeting